Products
Search and purchase from millions of reagents, consumables and lab supplies listed all in one place.
Our digital research marketplace helps scientists carry out more innovative experiments in less time and at lower cost
Request a demoUse our award-winning marketplace to quickly find the tools and technologies you need
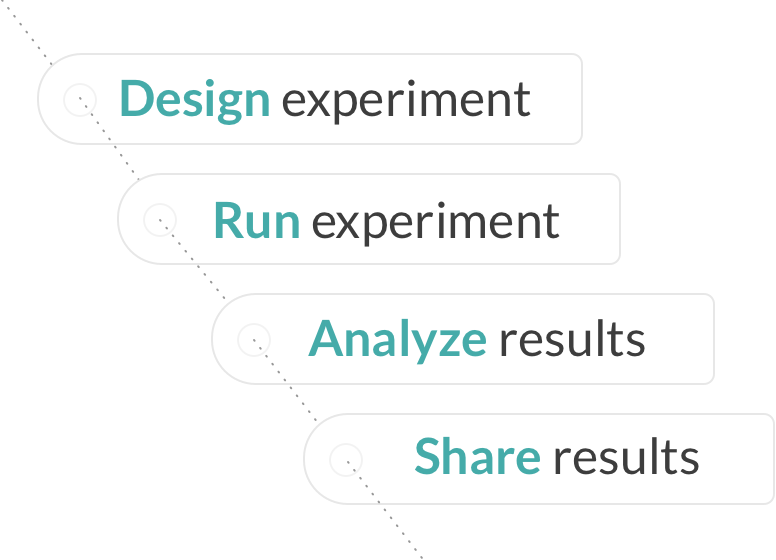
Test more ideas in less time and increase your chances of making new discoveries
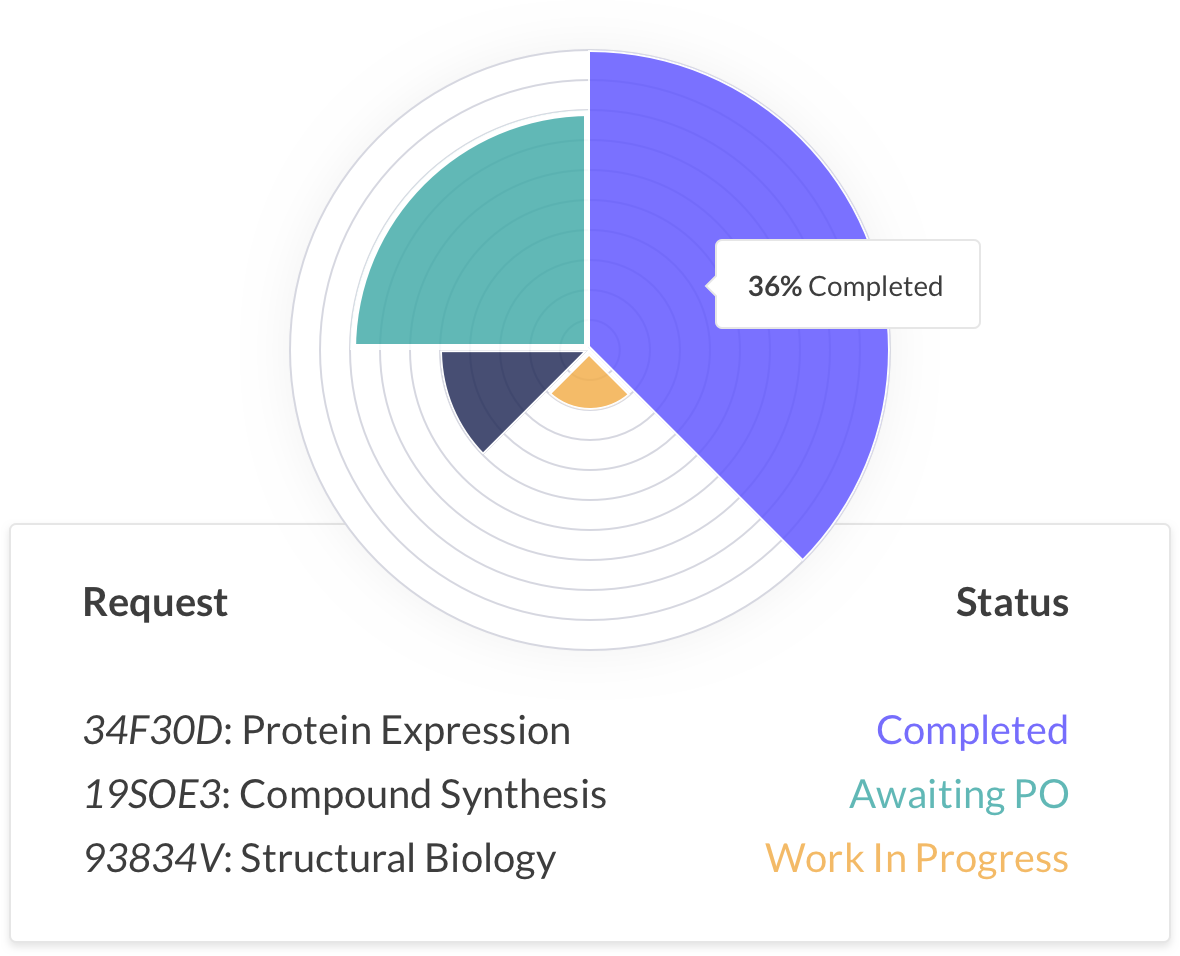
Automatically track purchases, manage legal contracts, negotiate prices and handle payments
Use machine learning algorithms trained using proprietary datasets to find the best supplier match
Learn moreOur partners include most of the world’s top pharmaceutical companies and a network of over 4,000 CROs